RBN-3143 – Reducing Th17 and Th2 signaling to treat multiple inflammatory diseases through PARP14 inhibition
Ribon’s second clinical program is RBN-3143, a first in class orally delivered small molecule inhibitor of the monoPARP PARP14, for the treatment of inflammatory diseases. Selective inhibition of PARP14 leads to a decrease in alarmin cytokines and dampening of the IL-17 and IL-4/13 signaling pathways. We have demonstrated efficacy in multiple preclinical models of inflammatory disease, including atopic dermatitis, scleroderma, ulcerative colitis, and asthma.
We are currently conducting a Phase 1 clinical trial with RBN-3143 with an initial focus on patients with atopic dermatitis. The study is designed to evaluate safety, as well as the pharmacokinetic and pharmacodynamic properties, of RBN-3143 in healthy volunteers and patients with atopic dermatitis.
For more information, go to www.clinicaltrials.gov or contact us at [email protected].
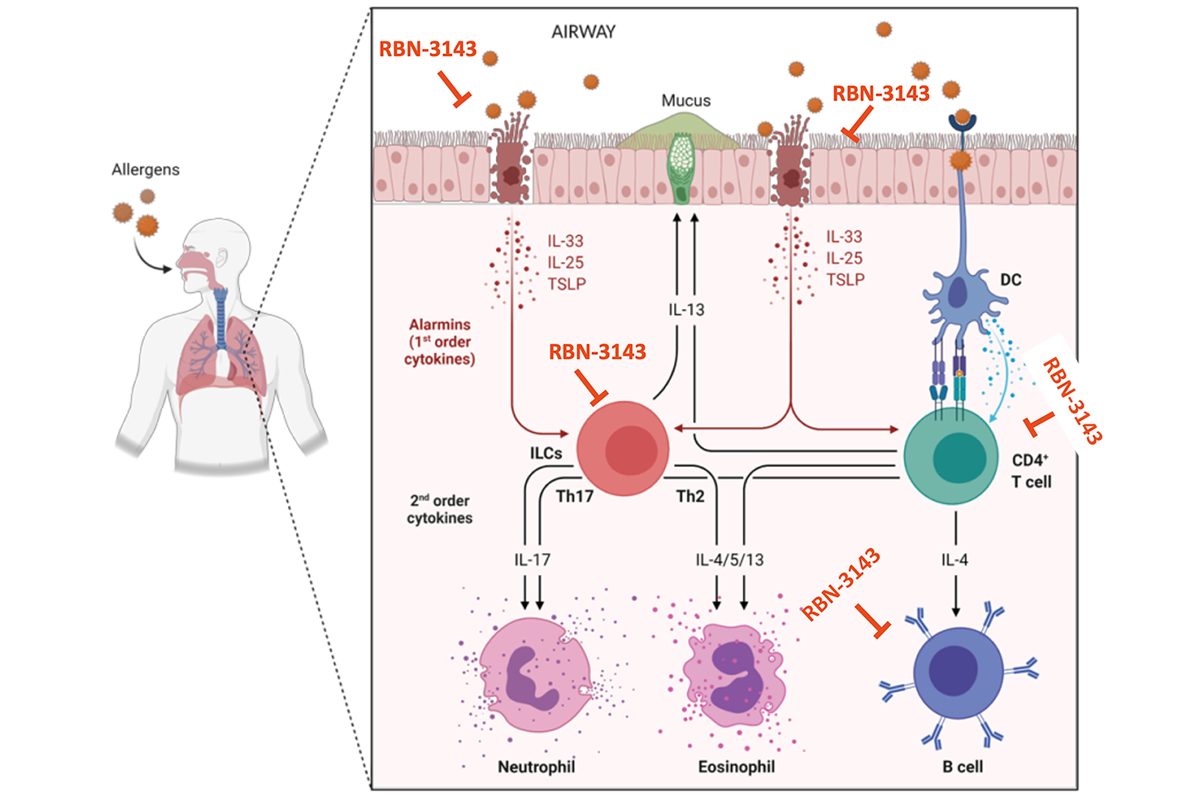
PARP14 is highly expressed in tissues of inflammatory diseases (and not constitutively in normal tissues), leading to the increase of first order cytokines (alarmins) and second order cytokines (Th2 and Th17 cytokines), and ultimately the increase in tissue eosinophils and neutrophils. Targeted reduction of these inflammatory pathways with RBN-3143 is anticipated to have improved efficacy over current therapies, such as those for asthma that only target single cytokines such as the IL-4, IL-5 and/or IL-13.